What Are Clinical Trials and Why Are They Important?
As a fundamental component in the advancement of medical knowledge, clinical trials play an important role in the discovery of new treatments, therapies, and procedures for diseases and conditions. They also help to find new ways of reducing, diagnosing, and detecting the chance of developing those diseases or conditions.
These studies are designed to show what works and what doesn’t in humans, key information that can’t be learned in the lab or with animal models. Think about all the current medications, therapies, and devices used to treat diseases and conditions today. You may not realize that they’re only available because they’ve gone through this rigorous process and had human participants involved in their clinical trials.
The ultimate goal in clinical trials is to improve health outcomes. For people living with a rare disease like acromegaly, clinical trials can provide an opportunity to try new therapies that aren’t yet approved (these are called investigational). Participation is also a chance to engage more frequently with physicians, to contribute to research that could ultimately improve their own condition, and to help others in the future.
Acromegaly treatment • 24 hours • 1 dose
The PATHFNDR studies are randomized, placebo-controlled, multi-center studies to evaluate the safety and efficacy of ONCE-DAILY, ORAL paltusotine in subjects with acromegaly. If you’d like to know more about these studies of this investigational drug, click here for more information and eligibility requirements.
Brain power. It can mean getting good grades or having a knack for figuring things out quickly. But in more literal terms it simply means getting the brain the energy, or in this case sugar (glucose), it needs to function. This sounds simple enough, but there are many things that can go wrong with the steps involved in delivering the right amount of glucose to the brain.
When something goes wrong with those steps, the brain can’t do its job. This may be the case for patients who have congenital hyperinsulinism (HI), a very rare disease involving the levels of glucose and insulin in the body, and how the body does – or doesn’t – regulate these levels. Basically, glucose is a nutrient and insulin, which is made by the pancreas, is what the body needs to use this glucose. Glucose and insulin normally work together to keep the glucose levels “just right.” When glucose goes up (like after a meal), the pancreas secretes insulin to return it to normal levels, and when glucose levels decrease insulin secretion is suppressed. Insulin also tells the rest of the body “I’m fed” so the body stores energy in the muscle, in the liver and in fat, rather than tapping into those reserves to give energy to the brain.
Life with HI is like a roller coaster ride, sometimes you’re high; sometimes you’re low but with research, funding, and new developments you never run out of hope.
— Jilani's parent
About Clinical Trials
There are different types of clinical trials, depending on what researchers are studying. Each is crucial in modern, evidence-based medicine in finding new treatments that could help improve a person’s health.
Preventive Trials
These typically include healthy individuals as well as people who previously had a certain condition and are trying to prevent it from coming back. Testing includes new medications, vitamins, minerals, supplements, vaccines, and/or lifestyle changes that might lower a person’s risk of certain diseases or conditions.
Screening Trials
Screening trials attempt to find the best ways to detect certain diseases or conditions before patients start to experience symptoms. The goal is often to determine if earlier detection of illnesses decreases a person’s risk of serious harm or death.
Diagnostic Trials
These trials aim to discover new ways to diagnose and detect certain medical conditions, and to improve testing methods, effectiveness of procedures, and the tools involved. Diagnostic trials usually include participants who are already experiencing signs and symptoms of the condition being studied.
Treatment (Intervention) Trials
These focus on finding new treatments for an array of diseases or conditions by collecting data and assuring the safety of participants, while also evaluating the drug or device. They involve an intervention such as drugs, existing drugs, or new combinations, psychotherapy, a new device, a new approach to surgery, or another type of therapy or treatment.
It is just as important in treatment trials to test drugs and medical products on the people they are intended to help as it is on a diverse variety of people. For example, patients who have been diagnosed with acromegaly are the perfect candidates for participating in clinical trials whose goal is to study a new treatment to control symptoms of acromegaly.
Therapeutic Trials
These investigate a treatment, not necessarily pharmacological, believed likely to benefit participants receiving the intervention.
Non-Therapeutic Trials
These types of trials are unlikely to produce direct benefits to participants, but strive to obtain knowledge that may contribute to the future development of treatments and procedures.
Genetic Studies
Genetic studies aim to improve the predictors of certain diseases or conditions by identifying and understanding how genes may play a role in certain illnesses. This is important in determining various treatments.
Quality of Life Studies
This type of research evaluates a person’s comfort and quality of life when suffering from a chronic illness. This study can also determine the best ways of managing the side effects of various diseases or conditions and treatments, including nausea, vomiting, and depression.
Epidemiological Studies
Research obtained in this type of study helps scientists identify patterns, causes, and control of different disorders in various groups of people.
Observational Trials
An investigator will observe certain groups without any intervention or manipulation. The purpose is to provide insight into diseases as they naturally occur, and the current therapies intended to treat them. Researchers may collect data that can be helpful for future research.
These terms describe different ways trials can be designed and conducted.
| TERM | DEFINITION |
| Subjects | Volunteers who take part in the study (also called participants or patients) |
| Randomized | Subjects are assigned to different groups by chance |
| Open-label | Subjects and researchers are all aware of the intervention being given |
| Single-blind | The subjects do not know which group they are in (e.g., whether they are in the treatment group or the placebo group) |
| Double-blind | Neither the researchers nor the subjects know which group the subjects are in until it is revealed at the end of the study |
| Placebo | A substance which does not contain the active ingredients of the experimental drug, but looks the same |
| Add-on | All subjects receive an existing medical therapy, but some then receive the additional experimental drug while others do not or are given a placebo |
| Single-center | The study is carried out at one location |
| Multi-center | The study is carried out in several locations (i.e., different cities or even different countries) |
Randomization and Blinding
Researchers are committed to avoiding bias in clinical trials. Bias refers to human choices (either intentional or unconscious), or other factors that might affect the results of the trial, such as selecting which patients to assign to certain comparison groups.
In treatment clinical trials, research that compares interventions or treatments commonly uses randomization as part of the study design. This means volunteers are randomly assigned to study “arms” or groups. Which intervention or “treatment” the volunteers receive depends on the study arm they’re assigned to.
Bias in clinical trials
Bias refers to human choices (intentional or unconscious), or other factors that might affect the study results, such as selecting which patients to assign to certain comparison groups.
For example, bias occurs if a doctor running a treatment trial assigns sick patients to the group receiving the intervention, and healthier patients to the control group that will receive the placebo. Such a bias can impact the results of the trial.
Researchers are committed to avoiding bias in clinical trials. A common way to accomplish this when comparing interventions or treatments is by using randomization as part of the design study. This means volunteers are randomly assigned to study “arms” or groups. Which intervention or “treatment” the volunteers receive depends on the study arm they’re assigned to instead of a conscious choice by those running the trial.
The Principal Investigator
Every clinical trial is overseen by a principal investigator (PI) , who is typically a medical doctor. The PI has expertise in study design, regulatory compliance, data management, project management, and statistical analysis. In a multi-site trial, a PI is typically assigned for each site.
The Sponsor(s)
Sponsors prepare the protocol for the trial and oversee the study. They are often pharmaceutical companies that develop the novel drug to be tested, but may also be physicians, hospitals, government agencies, institutions, advocacy groups, or other organizations.
The Protocol
Protocols are specific guidelines and procedures that detail how the trial will work, i.e., what happens during the trial, when, and why. They are in place to ensure the study results are reliable and help to reduce the risk to participants. Wherever the clinical trial is conducted, the same protocol is strictly followed. It might contain:
- An explanation of the goal of the study and intention
- Number of participants
- How long the study will be
- Eligibility (i.e., inclusion and exclusion criteria such as sex, age, type and stage of disease, current
and/or previous treatment history, and the presence of other medical conditions. Not every person
with the disease or condition being studied will qualify for the trial. - Specific tests to administer and how often
- The type of data to be collected
Healthcare Professionals
A team of credentialed, qualified physicians, nurses, physician assistants, and pharmacists who monitor all participants per the protocol and record the findings.
The Volunteers
The subjects participating in the study. Depending on the phase of the study and the type of study being conducted, these can include healthy individuals or patients diagnosed with the condition or disease being studied.
The Study Site
The place where a clinical trial or study is conducted, which must meet criteria set forth by regulatory agencies.
Inpatient/Outpatient Studies
Inpatient studies are conducted with participants who are admitted to a hospital or other facility where they get 24-hour care and observation. In outpatient studies, patients are not admitted but they are periodically examined by the study team to record/observe parameters outlined by the study protocol.
The Independent Review Board (IRB)/Independent Ethics Committee (IEC)
This group assures appropriate steps are taken (before and during the study) to protect the rights and welfare of all participants. They check to see the trial is well-designed, does not pose undue risks, and includes safeguards for patients. The IRB must approve the action plan for every clinical trial, and one is assigned to each study site.
Every drug development program begins in a lab, where scientists develop and test new ideas. This can include drugs, vaccines, and medical devices. If the results of these tests are promising, the study advances to the next step.
Pre-Clinical
Experiments are conducted with the goal of reaching a “proof-of-concept.” Researchers look at the drug’s nature, its chemistry, its effects (pharmacology), and its potential damage to the body (toxicology). Other measures include:
- How much of a drug gets absorbed into the bloodstream
- The potential toxicity of the product and how it’s broken down by the body
- How quickly the drug and its metabolites (the substances that remain after a drug is broken down by the body) are excreted
The underlying purpose in this phase is to determine a drug’s safety before it is studied in human subjects. This work continues throughout the development program.
From here, promising medical therapies typically pass through three phases of interventional clinical trials, as explained below. Actual trials may vary from the phases outlined here, depending on the type of trial and the intervention (drug) and/or disease being studied.
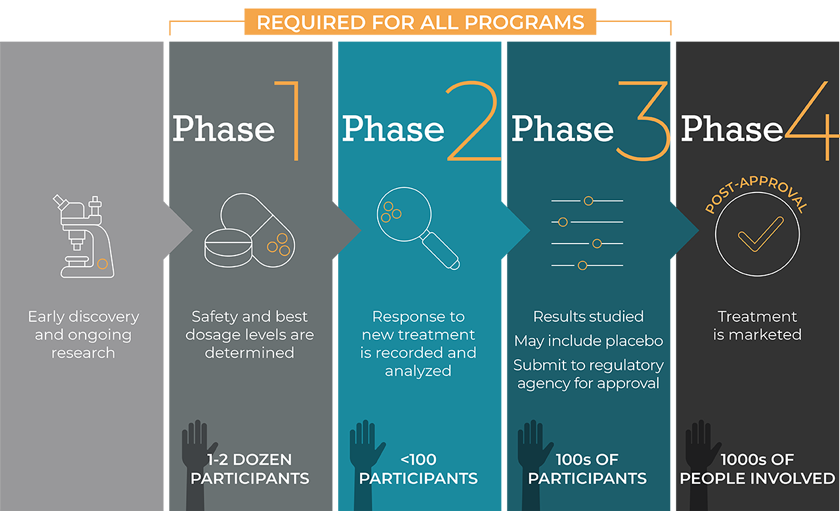
Phase 1
Small, usually enrolling less than 30 healthy volunteers. Phase 1 trials are designed to find a safe dose of the new treatment, determine how the treatment should be given, and learn how it affects the body.
Phase 2
Small, usually enrolling less than 100 participants who have the disease/condition. Researchers study how the treatment affects the body and how it works for a specific disease (for example, acromegaly). If the treatment is found to be safe and have some benefit, the study goes on to Phase 3.
Phase 3
Phase 3 studies the effects of the drug in a larger population of participants (hundreds, sometimes thousands) who have the disease/condition being studied. This sometimes includes the use of placebo.
At this point, the regulatory agency reviews the entire development program as well as the results of the clinical trials, and approves the new drug (or not).
Phase 4
Phase 4 studies are not always required, and usually conducted to monitor a specific potential safety concern or a specific population.
There are almost as many reasons for participating in a clinical trial as there are people who do so. It’s a highly personal decision that most participants reach only after discussing it with family, friends, and their doctor(s). A few of the more common reasons people choose to do it include:
Helping to develop better treatment options
Some people are motivated to participate in a clinical trial because currently available treatment options haven’t worked, or certain side effects have a negative impact on their quality of life. Clinical trials provide a way to explore where current therapies fail or to identify areas of needed improvement.
Paying it forward
Giving back by way of contributing to the advancement of medical knowledge is another reason people participate.
A PATIENT’S VOICE
New treatment options would mean more freedom instead of the same old shot every month. It would give everyone hope that they’re working on something, and maybe someday a cure.
— Acromegaly patient
How Clinical Trial Participants Are Kept Safe
All clinical trials come with risks, as does any medical procedure. It’s common that the safety and efficacy of a new drug or other therapy may not be fully known at the start of a clinical trial. In addition, some trials may give participants medical benefits, while others cannot. The question is whether the potential benefits outweigh the risks. Here are some ways in which the risks can be explained to participants:
Informed Consent
Informed consent involves more than simply consent to participate. This is because trials often require participants to go through additional procedures, tests, and assessments based on the study protocol. All of these risks and requirements must be presented, understood, and accepted during the informed consent process prior to the start of the research.
Written or verbal informed consent is only one step in the process:
- Volunteers for clinical trials are given materials and information to allow for an informed decision on whether to participate. They are encouraged to discuss any potential risks with their doctor before beginning the trial.
- Potential participants are evaluated to ensure they can understand the information presented to them, and given time to fully digest and consider it.
- Formal consent is obtained indicating each volunteer’s agreement to participate.
- Information is continually provided to all participants as the trial progresses and/or as the need arises.
Careful, continuous monitoring
Each trial is reviewed by an ethics committees (also called Institutional Review Boards, or IRBs), as well as by regulatory agencies (in the US, this is the FDA) to ensure patient safety stays the top priority.
Safe Withdrawal
Even after providing consent to participate, participants can withdraw from the study at any time. Possible reasons for doing so include if they experience unpleasant side effects, if the treatment doesn’t work, or if they no longer want to be a part of the trial.
Rescue Procedures
Especially in interventional trials in which participants are given a drug, they are closely monitored for any signs of a decline in health. Should this be the case, “rescue” procedures may be in place to ensure appropriate therapies are provided. These can be existing treatments, and could be the medication investigators were trying to improve on in the first place.
The Final Word
The most important aspects in the discovery of new treatments are its safety and effectiveness. Clinical trials pave the way for the development of medical advancements and improved therapies. Patients and families affected by rare diseases like acromegaly know all too well the challenges of managing symptoms and quality of life where treatment options are scarce, inconvenient, and/or painful.
If you’re considering participating in a clinical trial, your doctor may be able to suggest an appropriate study for your condition. We also encourage you to explore the resources on this page to learn more.
Subscribe
The Better Bulletin, our periodic newsletter, helps you stay informed on our work to improve the lives of patients living with rare endocrine diseases.
Resources
ClinicalTrials.gov
This database of privately and publicly funded clinical studies conducted around the world is one of the best resources available. You can search by disease, country, and other key filters to find a trial that may be right for you.
The site includes a good list of questions to ask when considering participating in a clinical trial. See them here.
Why participate in clinical research?
This video features researchers and clinical trial participants talking about what it’s like to volunteer for a trial and how it promotes medical advances.
Deciding to participate in clinical trials
Animation and easy-to-understand language make this video from the Office for Human Research Protections (OHRP) a must-see for any potential trial participant.
Another OHRP video addresses randomization. Watch it here.
A participant’s first-hand account
Watch Juliana’s story about her experience participating in a trial for sickle cell anemia.
Informed consent for clinical trials
This page from the FDA website has a lot of good information on the topic.
training/clinical-trials]. Retrieved May 2020.
[https://www.fda.gov/patients/drug-development-process/step-3-clinical-research].
Retrieved May 2020.
[https://clinicaltrials.gov/ct2/about-studies/learn]. Retrieved May 2020.
[https://www.nhs.uk/conditions/clinical-trials/]. Retrieved May 2020.





